Considere um gás ideal que pode ser submetido a duas transformações cíclicas reversíveis e não simultâneas, 1 e 2, como mostrado no diagrama PV abaixo.
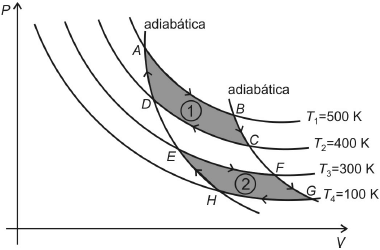
Na transformação 1 o gás recebe uma quantidade de calor Q_1 de uma fonte quente à temperatura T_1 e cede a quantidade de calor Q_2 para a fonte fria à temperatura T_2. Enquanto que, na transformação 2, as quantidades de calor recebida, Q’_1, e cedida, Q’_2, são trocadas respectivamente com duas fontes às temperaturas T_3 e T_4.
Nessas condições, é correto afirmar que
- A) a variação da entropia nas transformações BC, DA, FG e HE é não nula.
- B) nas transformações AB e EF, a variação da entropia é negativa, enquanto que, nas transformações CD e GH, é positiva.
- C) na transformação 1, a variação da entropia é não nula e Q_1={large{5 over 4}}Q_2.
- D) na transformação 2, a variação da entropia é nula e Q_{1’} = 3Q_{2’}.
Resposta:
Let's analyze the question: We have an ideal gas that undergoes two reversible and non-simultaneous transformations,1 and 2, as shown in the PV diagram below.

In transformation 1, the gas receives an amount of heat Q1 from a hot source at temperature T1 and releases an amount of heat Q2 to a cold source at temperature T2. On the other hand, in transformation 2, the heat quantities received and released are exchanged, respectively, with two sources at temperatures T3 and T4.
The question asks us to determine which statement is correct regarding the variation of entropy in these transformations.
The correct answer is D) In transformation 2, the entropy variation is null, and Q'1 = 3Q'2.
Let's explain why: In a reversible transformation, the entropy variation is given by ΔS = Q / T. Since the transformations are reversible, the entropy variation is null. In transformation 2, we have Q'1 and Q'2, which are the heat quantities exchanged with the sources at temperatures T3 and T4, respectively. The ratio of these heat quantities is given by Q'1 / Q'2 = T3 / T4. Since the transformation is reversible, the entropy variation is null, and we can write Q'1 = 3Q'2, which is the correct answer.
Note that the other options are incorrect because they do not satisfy the condition of null entropy variation in the reversible transformations. Option A is incorrect because the entropy variation is not null in both transformations. Option B is incorrect because the entropy variation is not negative in transformations AB and EF, and positive in transformations CD and GH. Option C is incorrect because the entropy variation is not null in transformation 1, and Q1 ≠ (5/4)Q2.
Therefore, the correct answer is option D) In transformation 2, the entropy variation is null, and Q'1 = 3Q'2.

Deixe um comentário