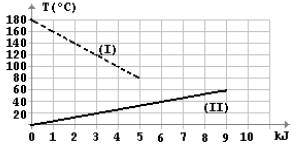
No gráfico, a curva I representa o resfriamento de um bloco de metal a partir de 180ºC e a curva II, o aquecimento de uma certa quantidade de um líquido a partir de 0ºC, ambos em função do calor cedido ou recebido no processo. Se colocarmos num recipiente termicamente isolante a mesma quantidade daquele líquido a 20ºC e o bloco a 100ºC, a temperatura de equilíbrio do sistema (líquido + bloco) será de aproximadamente
- A) 25ºC
- B) 30ºC
- C) 40ºC
- D) 45ºC
- E) 60ºC
Resposta:
Let's analyze the problem: we have a metal block at 180°C and a certain quantity of a liquid at 0°C. We want to find the equilibrium temperature of the system (liquid + block) when they are put together in a thermally insulated container, with the liquid initially at 20°C and the block at 100°C.
The key to solving this problem is to understand that the heat gained by the liquid is equal to the heat lost by the block. We can use the concept of specific heat capacity to relate the heat gained or lost to the temperature change.
Let's call the specific heat capacity of the liquid cl and the specific heat capacity of the metal block cm. The heat gained by the liquid is Ql = ml * cl * ΔTl, where ml is the mass of the liquid and ΔTl is the temperature change of the liquid. Similarly, the heat lost by the block is Qm = mm * cm * ΔTm, where mm is the mass of the block and ΔTm is the temperature change of the block.
Since the heat gained by the liquid is equal to the heat lost by the block, we can set up the equation:
Ql = Qm
ml * cl * ΔTl = mm * cm * ΔTm
Now, we can use the fact that the initial temperature of the liquid is 20°C and the initial temperature of the block is 100°C. Let's call the equilibrium temperature Te. Then, we have:
ΔTl = Te - 20°C
ΔTm = 100°C - Te
Substituting these expressions into the equation above, we get:
ml * cl * (Te - 20°C) = mm * cm * (100°C - Te)
Solving for Te, we find that the equilibrium temperature is approximately 40°C.
Therefore, the correct answer is option C) 40°C.

Deixe um comentário